About us
Welcome to the EIT Health Biobanks and Health Data Registries Portal.
EIT Health
At EIT Health, we are committed to advancing healthcare innovation and improving patient outcomes. Biobanks and health data registries play a crucial role in medical research, providing valuable resources for understanding diseases, developing new treatments, and tailoring healthcare approaches. Our platform serves as a centralized hub where researchers, clinicians, and industry professionals can access information on European biobanks and health data registers, enabling them access to these resources.
On this website, you will find profiles of biobanks and health data registries from across Europe, highlighting their characteristics, specialties, and available samples or datasets. Whether you are searching for specific samples, genetic information, or other health-related data, our platform will guide you towards the relevant sources and facilitate access to these resources.
We are proud to collaborate with leading biobanks and health data registries in Europe. By providing an accessible resource, we aim to accelerate scientific breakthroughs, innovation, and ultimately contribute to a healthier future.
Join the EIT Health Academy!
Our educational programmes cover a broad range of topics and are unique offerings that give you the opportunity to learn new skills, connect with a vast network of experts, access new markets and finance, whilst receiving accreditation for your studies. Our programmes.
Learn how to navigate biobanks – join the EIT Health Academy!
Whereas biobanks are of absolute importance to medical advancements their use is very limited due to unclarity on the legislation for material access and lack of awareness, amongst other reasons. Therefore we recently collaborated with BBMRI-ERIC and created a fully immersive course to educate prospective and current biobank users across the borders. Enroll in the course and receive accreditation. Here
Biobanks in Europe
BBMRI-ERIC is a European research infrastructure for biobanking bringing together the main players from the biobanking field – researchers, biobankers, industry, and patients – to boost biomedical research. BBMRI-ERIC offer quality management services, support with ethical, legal and societal issues, and a number of online tools and software solutions. Ultimately, its goal is to make new treatments possible. BBMRI-ERIC currently includes 23 countries and one international organisation (IARC/WHO), making it one of the largest European distributed research infrastructures.
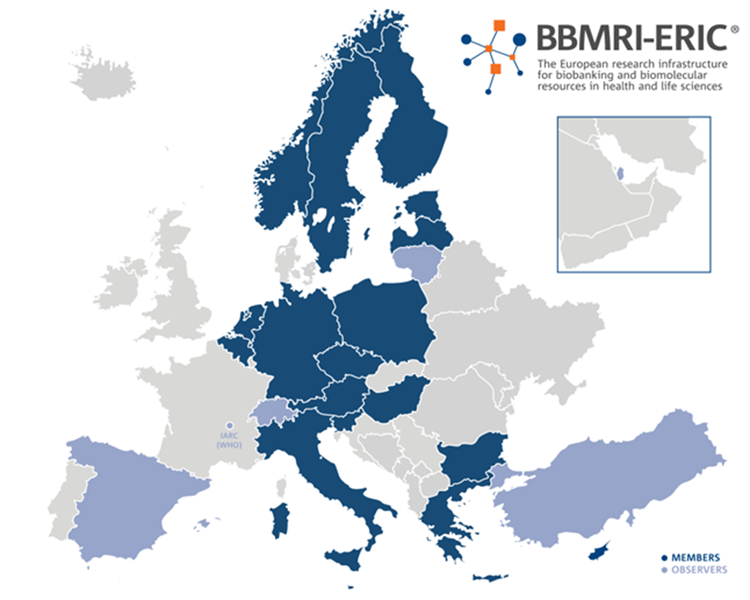
The mission of BBMRI-ERIC is to establish, operate, and develop a pan-European distributed research infrastructure of biobanks and biomolecular resources to facilitate the ACCESS to RESOURCES as well as FACILITIES and to support high-quality biomolecular and medical research.
Besides offering to biobanks quality management services, support with ethical, legal and societal issues, they offer a number of online tools and software solutions for describing the landscape of biobanks and sample repositories in Europe and facilitating access to these.
Access to European Biobanks which are a part of BBMRI-ERIC
The rules of access to biobank resources are determined separately by each country or biobanking unit. Quick and effective search of biobank resources and negotiation of access to samples is carried out using tools developed by the BBMRI-ERIC community, such as the Directory, Negotiator and Sample/Data Locator.
Directory
The Directory is a broad catalogue of our biobanks with aggregated information on their collections. It is tool that the user can utilise for finding suitable biobanks based on general criteria (diagnosis available, type of materials, countries, quality marks, collection types, data types). The database includes currently information from more than 400 biobanks with over 2900 collections, which together provide access to hundreds of millions of samples. BBMRI-ERIC Directory
Negotiator
BBMRI-ERIC provides its Negotiator platform to support researchers and biobankers in this complex communication, allowing effective streamlining and monitoring throughout the process. This is particularly useful as the requests become more complex (for example the user needs to access sample/data or communicate with multiple candidate biobanks) and biobanks and biomolecular resources also need time to verify availability of the data and biological material for a particular purpose defined by the researcher/requester. BBMRI-ERIC Negotiator
Sample/Data Locator
The Sample/Data Locator will be a service to locate samples and data sets hosted by the biobanks that are of interest for the requesters. The Locator will allow for detailed privacy-preserving, multi-criteria search of samples and data sets. This will also include the development of connectors to interface to the information systems of biobanks.
Access to samples of Rare Diseases and COVID-19
Biobanks from across the globe are invited to share their collections on the BBMRI-Directory to be visible to the entire research community throughout the world.
The European Health Data Space
The legislative proposal for the European Health Data Space (EHDS), published on May 3rd, 2022 by the European Commission, is a key building block for the creation of a sustainable and resilient European Health Union, and an unprecedented opportunity to leverage the European power to innovate. The EHDS creates a much-needed balance between the rights of patients to control and access their own data and the needs of healthcare professionals, providers, industry, researchers and decision-makers to use health data both for health care delivery and for public health, scientific and R&D purposes. Importantly, the ability for innovators to use high-quality health data is an indispensable requirement to transform health care and deliver more value to patients.
The EHDS framework has three key objectives:
1. To give individuals better digital access to their personal health data and to support free movement by having that data follow them across the Union.
2. To promote the data economy by fostering a single market for digital health services and products.
3. To set up strict rules for the use of an individual’s non-identifiable health data for research, innovation. policy making, and regulatory activities.
During the Swedish presidency of the European Commission 2023, EIT Health Scandinavia, in collaboration with Swedish Medtech, organised a round table to discuss the opportunities and obstacles that exist for the upcoming implementation of EHDS. The round table was one in a series of ten that took place across the EU during 2023, feeding into the final report to be launched in April 2024. The Swedish round table report is available for download here: Implementing the European Health Data Space in Sweden.
